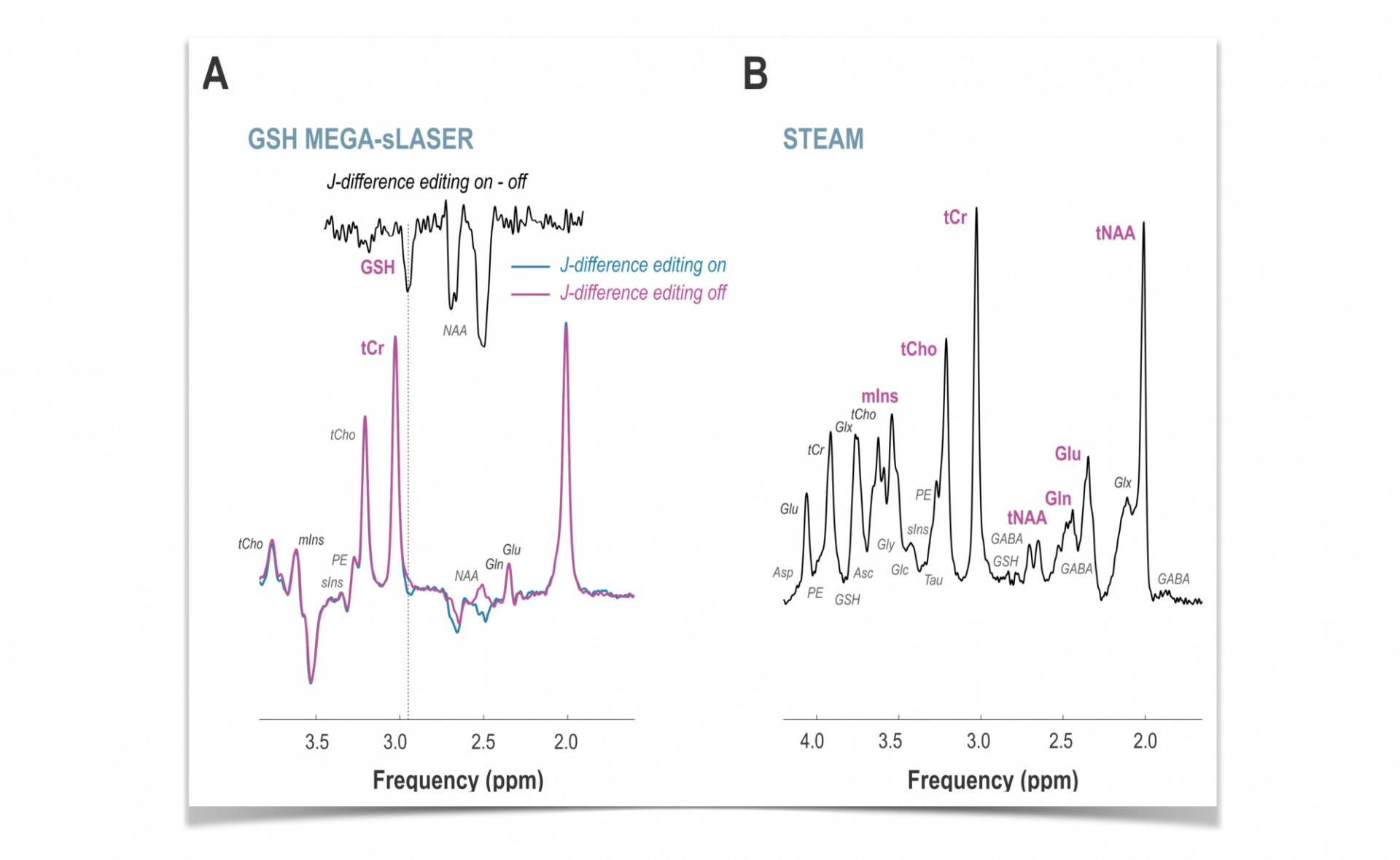
The MR SCIENCE Laboratory publishes its latest clinical research on “In vivo cortical glutathione response to oral fumarate therapy in relapsing-remitting multiple sclerosis: A single-arm open-label phase IV trial using 7-Tesla 1H MRS“ in collaboration with colleagues from Yale University and Biogen Idec in the journal Neuroimage: Clinical with additional support from the Yale-New Haven MS Clinic, the National Multiple Sclerosis Society (NMSS), the Nancy Davis Foundation and the National Institutes of Health (NIH).
Brain cortical glutathione concentrations are measured by 1H MR spectroscopy at UHF 7T at baseline before and during the use of oral fumarates as a disease-modifying therapy (DMT) for multiple sclerosis. The primary endpoint of this research was the change in prefrontal cortex glutathione concentration relative to a therapy-naïve baseline after one year of oral fumarate therapy. Among others, a general linear model demonstrated a significant positive linear relationship between prefrontal glutathione and time linearly across all time points in the multiple sclerosis cohort relative to baseline. No such effects of time on glutathione concentration were demonstrated in the healthy volunteer group. While the open-label single- arm pilot study design and abbreviated control series cannot support firm conclusions about the influence of oral fumarate therapy independent of test–retest factors or normal biological variation, these results provide first evidence for a relationship between brain glutathione increases and oral fumarate therapy in relapsing-remitting multiple sclerosis.
The open-access publication can be found here.
